Cancer immunotherapy aims to help the immune system find and destroy cancer cells. It can take many forms, from monoclonal antibodies and cancer vaccines to cell therapy. Emmanuel Savioz, CEO of Tigen – a biotech based at Superlab – tells us more about his vision for cell therapy and how collaborations between industry and academia could fast forward the development of new treatments.
The promise of cell and gene therapies
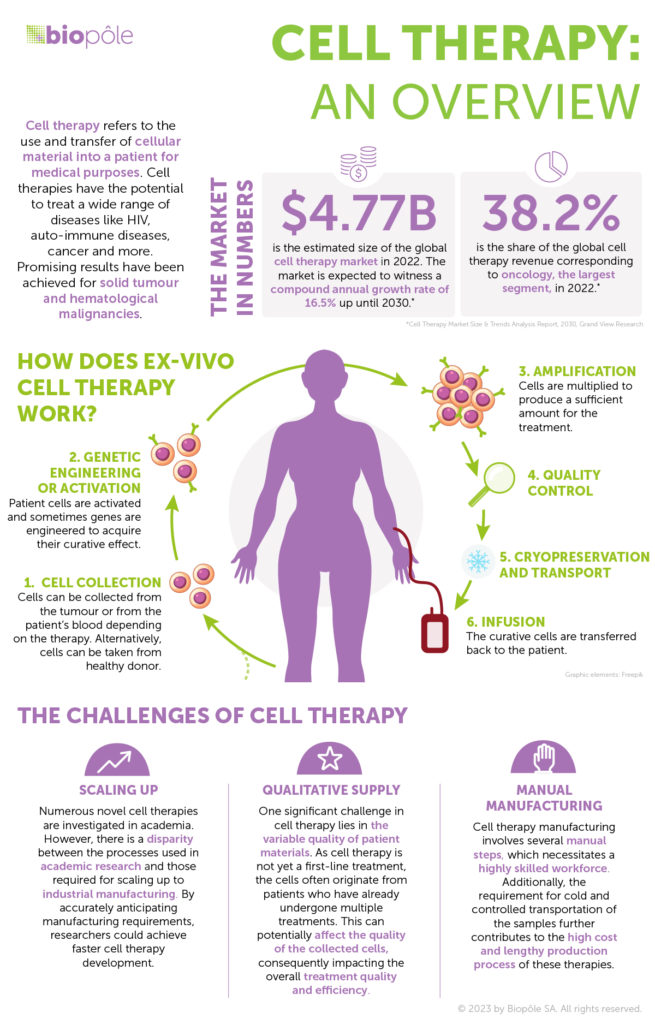
Cell therapy as a first-line cancer treatment is still a distant prospect. Today, approved cell therapies are used for patients with liquid tumours – like blood cancers – that haven’t responded to standard care or more conventional treatments. The results are promising, particularly for leukaemia, where remission has proven effective with just one dose. When it comes to solid tumours, cell therapy is still considered to be an experimental treatment: these tumours are better at evading attacks from the immune system and efficient treatments are therefore harder to develop.
But cell therapies to fight solid tumour cancers are coming out of infancy. Large-scale studies have shown very promising efficacy and superior results to other existing treatments. Cell and gene therapies are the early developments in a new era of precision medicine that is emerging from new collaboration models between academia, R&D, clinicians, manufacturing, biotech and pharma companies. The process involves shipping blood from the donor or patient and tumour samples to a production facility, priming the tumour-fighting cells, sending the treatment back to the hospital and injecting it into the patient.
Reaching more patients: the obstacles we need to overcome
Today, cell therapy treatments involve a complex, highly manual and lengthy development and manufacturing process, leading to costly treatments. The therapy is individualised, with no two treatments alike: each is created from scratch with the patient’s own cells to attack their specific tumour. At present, only the wealthiest nations can afford this kind of therapy to treat cancer. Even in the richest countries, there isn’t enough supply, with only a small fraction of patients able to access the treatment they need. The time it takes for a treatment to be ready also depends on the slots the pharma companies that manufacture and supply them have available. This can result in a life-threatening wait of several months. For a patient with an aggressive cancer who only has a few months to live, it can mean the difference between life and death.